Lenterra Inc. Launches a New Website
November 16, 2021 2022-01-04 18:12Lenterra Inc. Launches a New Website
Lenterra Inc. Launches a New Website
January 2022
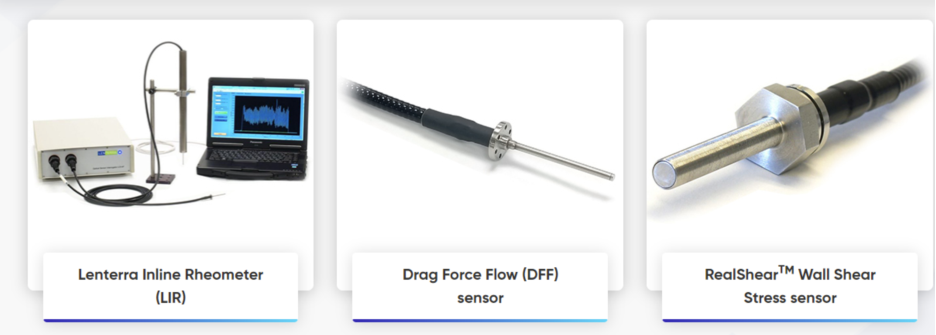
The new website from Lenterra Inc., a leader in inline rheometry, is a resource that presents multiple applications of its innovative products. Showcasing company’s library of white papers, it includes detailed explanation of Lenterra’s flow measurement technology and application examples for both powder and liquid flows. Company’s portfolio of flow force, shear stress and shear rate solutions is presented with detailed descriptions of how they address processing issues, for various industries such as pharmaceutical, bio-tech, food, and others including hydrocarbon transportation. These solutions include the flagship product - Lenterra Inline Rheometer (LIR) of different configurations - for comprehensive real-time process monitoring and material characterization.
“Our website is much more than simply product information,” said Vadim Stepaniuk, Director of Operations, “and I would definitely recommend it to everybody who are interested in flow characterization solutions. I hope it will become a valuable resource for processing engineers and researchers.”
About Lenterra
Lenterra Inc., a New Jersey, USA corporation was founded as a developer of innovative micro-optical sensing technologies for wall shear stress measurement in gases and liquids, and after 2012 expanded his portfolio to powder characterization and powder flow measurements. Company’s flagship product – Lenterra Inline Rheometer (LIR) - is a unique instrument that employs patented Drag Force Flow (DFF) sensor and is capable to continuously monitor flow force, uniformity and temperature during processing. The company invests significantly in R&D and applications development towards inline process instrumentation. With several years of experience, Lenterra is well qualified to address user’s individual flow characterization challenges, focusing on delivering the most relevant solution for in-process material characterization, formulation development, scale-up, quality control, or other areas.

